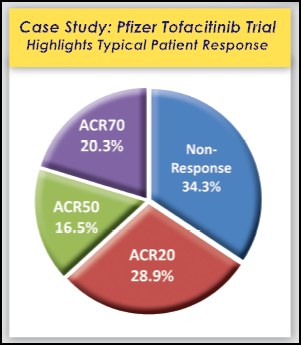
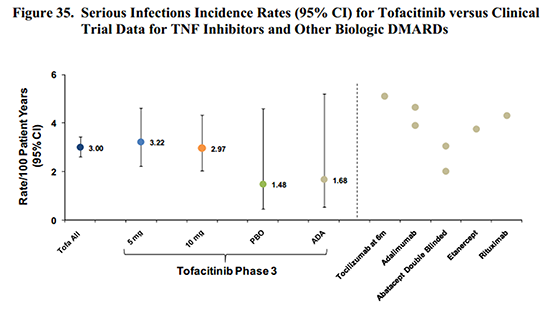
An Unremarkable Reality: Scans Don’t Lie – or Do They?
Show me your erosions or else
Some of you may remember last year when we met Dr. Shrug who gave my foot an ultimatum: Show me a bone erosion, or no more DMARDs. We only ever saw the doc once (more than enough). It was Dr. Tylenol’s partner, who they scheduled us with since Dr. T...Continue reading 57 Comments »
Read more