Xeljanz Rejected by European Medicines Agency
EMA rejects Xeljanz authorization
Late yesterday, the European Medicines Agency (EMA) rejected the Pfizer’s application to market Xeljanz (tofacitinib). The EMA application was refused because the Committee for Medicinal Products for Human Use (CHMP) “adopted a negative opinion” on approving Xeljanz, stating they were unconvinced its risks outweighed benefits. Last November, the FDA approved the 5mg dose of Xeljanz in the U.S. for “moderate to severely active rheumatoid arthritis” after “inadequate response or intolerance to methotrexate.” Tofacitinib has also been approved for use with Rheumatoid patients in Japan (5 mg) and Russia (5 and 10 mg).
Today, I spoke with Andrew Koenig, Pfizer’s North American Inflammation/Immunology Group Leader, about the decision. He said, “While they (CHMP) believed in the effect for signs and symptoms, they were unsure about the benefit with structural damage. And they were concerned about infections.” The EMA document concerning authorization of Xeljanz acknowledged “Xeljanz resulted in an improvement in the signs and symptoms of rheumatoid arthritis and the physical function of patients.”
Koenig indicated that Xeljanz is approved in the U.S. to treat “signs and symptoms” of RA, but not for structural damage: “We are working with the FDA to expand its label over time.”
Excerpt from the EMA refusal of Xeljanz
What evidence of risks and benefits did the CHMP consider?
“We do know this submission was the same as to FDA,” Koenig told me. He confirmed the same data were used for approval in Japan, Russia, and the U.S. The best way for the public to view that data is in the briefing document submitted in a public hearing at the FDA last May.
RISKS
If you view the document, it’s important to recognize which data apply to the 5 mg dose.
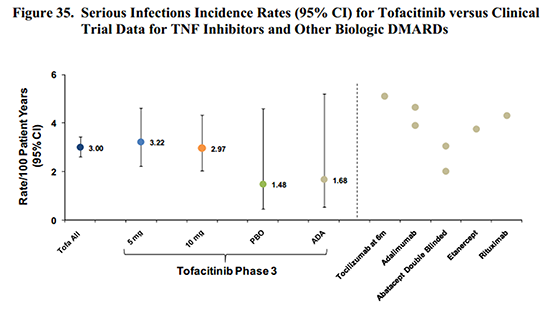 Several pages of charts cover various types of infection risk. While the disease itself is known to increase infections, the risks of the drug must be identified. Therefore, detailed infection data is presented for the nearly 5,000 patients included in the different clinical trials, which shows an increased infection risk with Xeljanz. As with other disease-modifying antirheumatic drugs or immunosuppressants, as well as prednisone, there were several types of infections encountered. Age groups are specified because infection risk is greatly increased in the over-65 age group, independent of any disease or medication.
Several pages of charts cover various types of infection risk. While the disease itself is known to increase infections, the risks of the drug must be identified. Therefore, detailed infection data is presented for the nearly 5,000 patients included in the different clinical trials, which shows an increased infection risk with Xeljanz. As with other disease-modifying antirheumatic drugs or immunosuppressants, as well as prednisone, there were several types of infections encountered. Age groups are specified because infection risk is greatly increased in the over-65 age group, independent of any disease or medication.
While it is easy to determine that an increased infection risk exists, it is a complicated question. For example, while tofacitinib risk seems comparable to adalimumab (Humira) or other biologics in some circumstances, Table 31 shows that people who take steroids with tofacitinib had a much higher infection incidence. And in some cases, the lower dose of tofacitinib showed higher risk. Even looking at it in black and white, the risk is easy to recognize, but not as simple to calculate. (I’ve read the sections on the other risks several times; but it’s too much to cover here in one post, and the dilemmas are similar.)
BENEFITS
The CHMP held that improvement in “signs and symptoms” and “physical function” was seen, but there was not consistent evidence of prevention of structural damage. Figure 18 of the FDA briefing compares the treated patients to placebo patients. While the effect looks obvious, it was only statistically significant in some groups.
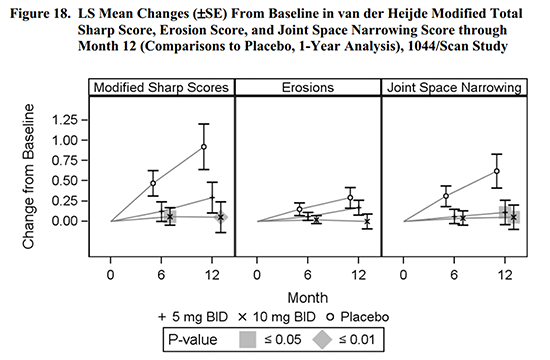
A company’s view of benefits of treatment
The executive summary of the Pfizer FDA briefing provided a discussion of Rheumatoid disease in order to portray the “unmet need,” an important argument in why any new treatment should be approved: “Because RA is currently incurable, the goals of treatment are to reduce disease activity, improve physical function and health-related quality of life and inhibit progression of structural damage throughout the course of the patient’s disease.” I agree with this broad focus.
In the section called “Summary of benefit-risk,” we read, “Up to one-third of RA patients receiving currently approved DMARD therapies show inadequate response in this serious, chronic, progressive and disabling disease that lasts 20 to 40 years and for which there is no cure.” It’s true that one-third of patients are “non-responders,” but remember that the FDA bar for “response” with RA is only a 20% improvement. I’m not sure how anyone’s Rheumatoid disease expires in 20-40 years except by death. I doubt that’s what they meant, but I cannot fathom where they got such an idea.
Risks and benefits from the patient point of view
Two things are missing in this discussion.
First, patients. The CHMP did not allow an opportunity for public comment, nor does it publish minutes or agendas. That is different from the FDA, which allows citizens like me to apply to speak at committee hearings, publishes agendas, and provides a live video feed of meeting activities.
Second, a disease perspective.
Last night I wondered who wrote the useless definition of RA in Pfizer’s press release: “About Rheumatoid Arthritis: RA is a chronic inflammatory autoimmune disease that typically affects the hands and feet, although any joint lined by a synovial membrane may be affected.”
This lack of communication of disease impact is partially responsible for thoughtless media coverage about their product and I would be surprised if it doesn’t impact people who make decisions about approving medications. Sometime this year, I hope you’ll be able to read my first book on Rheumatoid disease. When I look at the 200 footnotes, I see that if the evidence is there for a lay researcher like me to gather, it’s certainly available to Pfizer with its resources.
People with rheumatoid disease always have to weigh risks & benefits
My only question is whether authorities, the public, or perhaps even companies who create medicines, recognize what the disease impact is so that they can weigh risk to benefits accurately on our behalf.
People encounter risks every day that are higher than the risks with DMARDs: smoking increases cancer risks; obesity increases diabetes risk; tanning beds still find customers. But people faced with a harmful chronic disease have to choose whether to take certain risks with medications that may enable them to continue be functional, active members of society, or possibly live longer, when the medications work well. The risks should be clear, but many people with Rheumatoid Disease will choose to take them, because they see the alternative is so much worse.




Interesting post. I agree, they failed big time by not speaking to the patients. We need to be able to make the choice. Currently, I am making the choice of taking Xeljanz, and frankly I am starting to “live” life again…… Kim Byrne
I have been taking Xeljanz for 5 months. I’ve tried EVERYTHING ELSE through the years. My blood work and interleukin 6 and TNF factors are now normal. My IL6 was off the chart before taking this drug. I just do not get it! Read the contraindications of all the RA drugs – horrific side effects of infusions and other meds and you have to put it down they are so negative. Xeljanz is no more than others.
Once again, going back to the ignorance about RA, if I hear another person say “oh rheumatoid arthritis. I have that too , referring to their own “old age” arthritis symptoms, I give up. Change the name. And since when is this disease concentrated on hand areas? I’ve already had a shoulder and hip replacement and now need another hip AND a knee replacement from years past inflammation damage. I thank god for Xeljanz for my blood tests have never looked better and my fatigue is gone as well. For those who have given up with other meds this drug is meant to stop the cytokines inflammation at the hub – something other drugs including the dreaded infusions did not do for me. They all failed eventually.
Every time I had an infusion I never knew what type of reaction I was going to get. Taking 2 tablets a day is a far cry from what many of us have gone through! I hope others speak out about the benefits of Xeljanz for without it I would once again be in total despair.
Kelly- very interesting article. I couldn’t agree with you more– benefits complete out way the risks in many cases. Also– i learn more about my disease than any website or Dr through your site– and it makes me feel a little more “normal” and understood! On a side note I’m so happy you are writing a book – I can’t wait to read it!
We always have to weigh the benefits and risks…they should have talked to patients. We are often desperate for something, ANYTHING, to help even a little bit.
Xeljanz did nothing for me, it was like taking nothing, I flared incredibly badly on it. But after reading possible side effects it was MY choice to take the risk, as nothing else was working at the time.
That’s exactly right. And there are many who do respond to it – patients make the choice.
It’s also obvious whether there is a response within a short time period. Often patients endure side effects for several months before a decision is made that they do not respond to a biologic.
Is anyone taking Arava, Predisone ,and Cimzia?
Very interesting. As one of the patients who tried numerous biologics without improvement, I would be willing to take more risk because I feel QUALITY of life is so much more important than the quantity of years lived. It is as if they are assuming that we are ignorant of the risks or not intelligent enough to understand them.
I think that is a very important point Dori. But I also think it’s unclear the risks are greater than with biologics or other dmards. The problem is that the burden of the disease itself is so great, and seems to be discounted by those charged with making these decisions on behalf of the public.
Mainly affecting the bones of the hands and feet? Currently, I’m seeing a cardiologist because of arrhythmias and other difficulties. He has discovered some mild effusion around my heart, something that he directly attributes to my rheumatoid disease. Fortunately, the effusion is not impeding my heart right now.
I agree, it’s an inadequate description, at best.
scary report at first. So thankful for your comments. I’ve been on it 3 weeks. Have felt nothing so far. Still hoping
That’s interesting news. Although choices are now slim for me, my rheumy did not want me to take Xeljanz for the very reasons that it was rejected in Europe – Lack of long term efficacy and safety data. It did go through a few hiccups during FDA approval but finally got approved. Thanks for keeping us informed.
Andrew
Hi Kelly,
I’ve been taking Pfizer’s tofacitinib (I call it ‘the JAK’) for over a year. I take 10 mg twice daily, as I’m in a clinical trial. The JAK has changed my life and I’ve noticed no ill side effects — unlike the horrific experience I had with Humira. And while I still have severe R.A., my quality of life is so much better and I’m very grateful that Pfizer has created, is supporting and continuing to research this drug. Thank you for your wonderful and caring website. I just told my sister about rawarrior.com, as she’s pretty sure that she has R.A. and is beginning the process of testing and diagnosis. It’s heartwarming to read the posts on your website by others with R.A., knowing I’m truly not alone in struggling with this chronic disease. My whippet just did his little howl thing he does when he has a dream — so cute and funny — he makes me smile. Blessings to you all.
Marlice…I’m starting xeljanz today. Very anxious.
It may be my misinterpretation of some of their health care systems, but European agencies seem to be reluctant to accept new medications that are extremely expensive and appear to be “me too” drugs (i.e. there are a few biologics out already). If that’s the case, then I’m not surprised.
It’s tough. I hate the idea of further adding the marketing coffers of whatever drug company, but it feels worth it if it works. It’s less expensive in the long run to have treatment that is effective now rather than going through years of trying medications that aren’t helpful.
Kellly, as always you make an excellent point. Understanding the clinical impact on patients is critical, and helps to determine the risk-benefit ratio. But also worth noting is that the FDA’s role was designed to protect patients, often from drug companies/Big Pharma, whose interests are in profits more than in helping patients. Ideally, the FDA has a range of panel members who understand the drug and disease, and will decide wisely. I don’t think this is always an easy call. Should a cancer drug with many potential toxic effects be allowed on the market if there is no alternative? Can you trust that all health care providers will inform patients of the risks? I am glad the FDA is there with experts who can help evaluate these issues, and as you so well point out, it is up to us, the patients, to explain the disease and its impacts, therefore better enabling the risk-benefit decision.
Also, after reviewing all possible side effects, because I am trying to decide what drug to try next, I do think that this drug has not only all the side effects of the TNF drugs, but also additional potential adverse effects on liver, cholesterol, and the risk of GI rupture. Similar to Actemra, maybe, but more than some earlier drugs. Doesn’t mean not to take it, but only to understand the risks.
My 19 year old son takes Xeljanz and can almost run! I still can’t say these words out loud without choking up. He was an active, athletic boy who lost his ability to play sports his senior year of high school. Walking was very difficult and his struggle to walk on stage to receive his diploma at his high school graduation less than a year ago was heartbreaking. He had tried other therapies that didn’t work for him and now, after four months on Xeljanz, he can almost run!
Like others have said, it’s about weighing risk and benefit. I have MS so the anti-TNF therapies have a risk that is particularly scary for us. For now, this takes away choices where there aren’t many. The day may come where my son will have to weigh therapy choices again and make another decision but I’m happy he will at least have one. The last thing we all need is fewer choices.
Has anyone had problems with insurance not paying for Xeljanz? I started it a month ago with a free sample from my Rheumatologist. I was better within a 5 day period. It definitely is my magic bullet. However, when I mailed my prescription in to my mail order provider, it was returned and I was told to go to my local pharmacy. Xeljanz was not on their formulary. My provider is CHAMPVA, which is a program for military families. So now I am forced to do without my Xeljanz or pay over $500.00 per month. I am on disability so that is a huge amount for me.
Jolee, have you called the patient assistance line to see what they would have you do? (1-855-493-5526) I just got it from the website.
Jolee, have you checked with your CHAMPUS office at the base nearest to you? I get mine through my Tricare for life . Tricare and Champus serve the retired community and the active duty community respectively.
Thank you Darby.
No I haven’t spoken with them. I will give them a call and let you know. I did go to the website for Pfizer and it basically said that they could not offer any assistance if you have any kind of prescription coverage. Thanks for the suggestion!!
I had prescription coverage and received assistance.
I have prescription coverage and got it as well. My dr and Pfizer went to united health care and advocated. I am surprised they started you and then stopped. My dr said that Pfizer doesn’t want to start you and stop you, so they have a “advocate” called XELSOURCE that goes directly to your insurance to get it covered, since it was “working” I would think they want you to take it (makes them look good) I would,also try and get your dr on board, my dr rallied for insurance to pay for it, even the drug rep can get involved. It is a WIN for Pfizer for you to use their medication and get relief. I am on day 6.. And I am shocked at how quickly it has started to work. I am not 100%, but a clear and definite improvement. My expectations are realistic… Kim
Xelsource is what I googled to get that number. I hope they can help Jolee.
Glad it works for you Kim!!
Michelle, I read your post as my son was diagnosed at the age of 20 and has been treating for 2 years now. We have not been able to find anything that has slowed the progression for him. It is so heartwrenching to watch and feel unable to help. His Dr. just gave him the option of Xeljanz or Humira. He has been on Prednisone for over 2 years, he is trying to get off it, but has been unsuccesful so far. He has tried Metho, Rituxin and Enbrel, nothing is working. We are trying to decide which route to take and your post is very encouraging. I wish there was more information for the young adults that are battling this.
Laurie- My son just left today to go back to school out of state so I’m feeling even more emotional writing this…growing pains of motherhood. Anyway, my husband picked our son up from school the 2nd week of May and recorded him “running” to meet him with his phone. My son’s “run” was more of slow jog/trot but it was SO much more than he had been able to do and we were all more than happy and so grateful. Fast forward only three months and he is able to RUN a mile in under six minutes! Running with proper form and he worked as a counselor at a child care center all summer and played with kids 8 hours a day. His rheumatologist says he can go off all his other medications because Xeljanz is working so well. Now, if he would just take the leap and let my 16 year old daughter start Xeljanz so she can get some relief….. she was dx in March and her RA has been quicker and harsher with progression than my son’s but we still have to jump through the appropriate hoops at the appropriate time and in the appropriate order.
I take humira.
Tired all the time and have many flair ups.
Not sure what to do?
There are other options available in Europe that are not available here. My husband was successfully treated in Austria for 3 weeks for sever RA (Ankylosing Spondilitis).
I am happy to share. Last Feb, and he is med free.